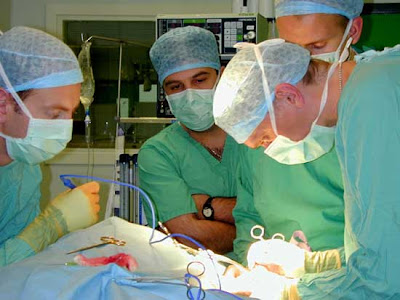
 Under the cover of blankets and the cloud of anesthetic, surgical patients give themselves over to doctors and nurses charged with their care.
Under the cover of blankets and the cloud of anesthetic, surgical patients give themselves over to doctors and nurses charged with their care.
Hours later, they may wake up in a recovery room without realizing someone other than the surgical team was present for their operation.
Representatives from medical device companies sometimes attend surgeries. They offer doctors technical advice on the heart pumps and orthopedic implants and a litany of other products they sell to hospitals.
In Calgary, patients are often unaware of the practice, in part because local hospitals don't require specific consent for a company representative to attend an operation, a fact some observers find troubling.
"You have to be really sensitive to people's privacy and their right to know who's involved with their care," says Dr. Guido Van Rosendaal, a physician and University of Calgary health policy researcher.
Experts say this is just one example of how the influence of device manufacturers and pharmaceutical companies in hospitals reaches further than many patients realize.
Industry's role in hospitals is at the centre of a national ethics debate after a recent Canadian Medical Association Journal article examined a B.C. health authority's new policy around sales representatives in its medical facilities.
The journal reported that Fraser Health brought in the rules after discovering some pharmaceutical sales people were paying surgeons an honorarium or educational grant to allow them to attend surgeries, a practice that is prohibited in Calgary.
Still, the changes at Fraser Health have some observers taking a close look at what industry practices are allowed in Calgary hospitals.
"There's a larger question in terms of what really is the role of sales reps in hospitals," says Barbara Mintzes, an assistant University of British Columbia professor, who studies pharmaceutical marketing practices. "Are some of these promotional activities leading to less appropriate patient care?" But the association representing Canada's research-based pharmaceutical companies argues their sales representatives have a role to play in medical facilities.
"We're making sure health-care professionals know the best information about prescription medications," says Russell Williams, president of Rx & D.
The debate follows the new Fraser Health policy, instituted this fall, that stops sales representatives from leaving samples of new drugs and other products at hospitals. It also bans salespeople from clinical areas such as operating rooms, unless they have permission.
The Calgary Health Region has a similar rule prohibiting pharmaceutical salespeople from meeting with physicians in any patient-care areas of hospitals. Representatives are not allowed to leave free samples of new products in CHR hospitals.
But Fraser Health's policy goes further than Calgary's, requiring vendors to receive an identification badge and "certification" when they arrive at a hospital.
The CHR has no such central intake process for salespeople, though officials concede some representatives occasionally wander into areas of the hospital where they are not allowed.
Steve Long, the CHR's director of pharmacy integration and strategic programs, says the health region contemplated introducing such controls, but decided against it because "we don't see there's an issue or problem." Calgary's policies also differ in one other significant way: CHR hospitals do not require explicit patient consent for a vendor to attend surgery, which is mandatory in Fraser Health hospitals.
Experts like the University of Calgary's Van Rosendaal say local hospitals should reconsider this practice.
CHR officials say vendors follow procedures to protect patient privacy and are only present in operating rooms to provide technical assistance.
"A vendor wouldn't be allowed into the room before a patient is draped and they talk about confidentiality," says Shanda Naylor, the CHR's director of perioperative services.
Officials with Rx & D, the association that represents the country's 50 research-based pharmaceutical companies, say they've banned gifts such as buying a round of golf for a doctor.
"We don't pay for access to medical professionals," says Williams of Rx & D.
Rx & D does permit companies to sponsor training activities for medical professionals and some Calgary doctors say they have little choice but to rely on such support for continuing medical education.
Dr. Debra Isaac, director of cardiac transplants for CHR, says sponsorships from industry allow her to hold training events where she can rent a venue, bring in speakers and even serve a meal. A session in rural Alberta, for example, might teach small-town doctors about treating heart failure.
Without the industry sponsorships, Isaac said, the events wouldn't happen.
"There's just no government funding, no hospital funding," says Isaac. "At this point, we'd be very limited without it." Experts like Mintzes say more public funding should be available to ensure continuing medical education is conducted at arm's length from pharmaceutical companies.
Mintzes also believes industry's self-regulation is problematic, arguing there have been cases where pharmaceutical companies paid for access to doctors, which is prohibited by Rx & D's code.
For now, however, it's left to industry and healthcare providers to find the right balance.
In Calgary, health officials say they have a strong set of rules in place to govern physicians' involvement with pharmaceutical companies.
Editor:
Steve Long, the CHR's director of pharmacy integration and strategic programs:"we don't see there's an issue or problem."
So don't we, after all: we're unconscious!


No comments:
Post a Comment